I-CATCHER
International Care Bundle Evaluation in Cerebral Hemorrhage Research (I-CATCHER)
a batched parallel cluster-randomized trial with a baseline period
2025
2025
A collaboration between Region Skåne, Malmö, Sweden and The George Institute, Sydney, Australia
2024
Ottawa Hospital Research Institute, Ottawa, Canada joins trial on the 20th of October
Iceland joins trial on the 20th of October
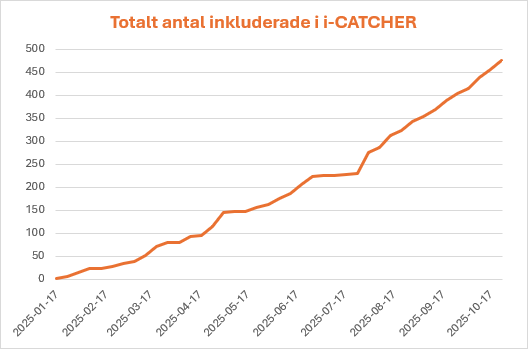
Rate of Inclusion
As of 251110
y-axis patient number, x-axis weeks
Background
Spontaneous intracerebral haemorrhage (ICH) accounts for approximately 10-15% of all strokes but stands for 50% of stroke-related morbidity and mortality. Approximately half of all patients with ICH have a decreased level of consciousness at hospital admission. For many years, randomized clinical trial (RCTs) have failed to establish a specific beneficial treatment after ICH. A study conducted in low- and middle-income countries has demonstrated a beneficial effect of a treatment package consisting of early intensive blood pressure lowering, as well as the treatment of pyrexia and elevated blood glucose levels. The I-CATCHER team is now planning to extend the study in Sweden and Australia, as well as in other high-income countries. The study has a clear focus on implementation, aiming to bring changes to clinical practice by integrating the care bundle as part of well-organised multidisciplinary care in stroke settings. The widespread adoption of the Care Bundle will optimise the chances of patients all over the world surviving free of major disability after suffering an ICH.
I-CATCHER will investigate whether a structured treatment package (Care Bundle) improves the 6-month prognosis of patients with spontaneous Intracerebral hemorrhage (ICH) compared to standard care.
Upcoming events
-
I-CATCHER Batch IV Start
November 17 2025
Sites launched in Australia, Canada, Italy, Malaysia, Hong Kong
-
European Stroke Organization Conference 2026
12th ESOC in Maastricht, Netherlands
6 - 8 May 2026